Clinical Focus ›› 2025, Vol. 40 ›› Issue (5): 439-444.doi: 10.3969/j.issn.1004-583X.2025.05.010
Previous Articles Next Articles
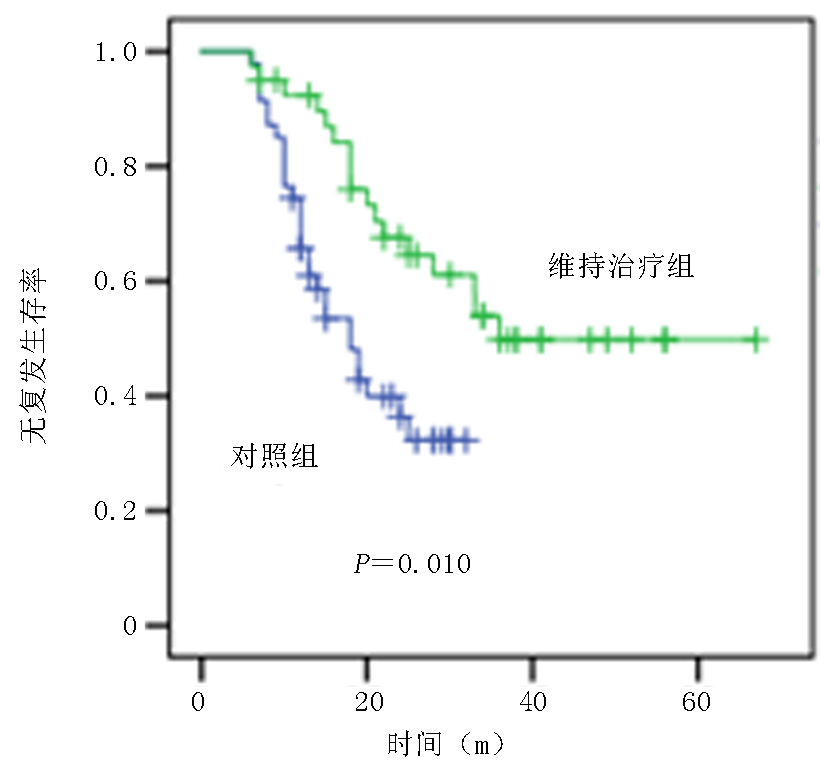
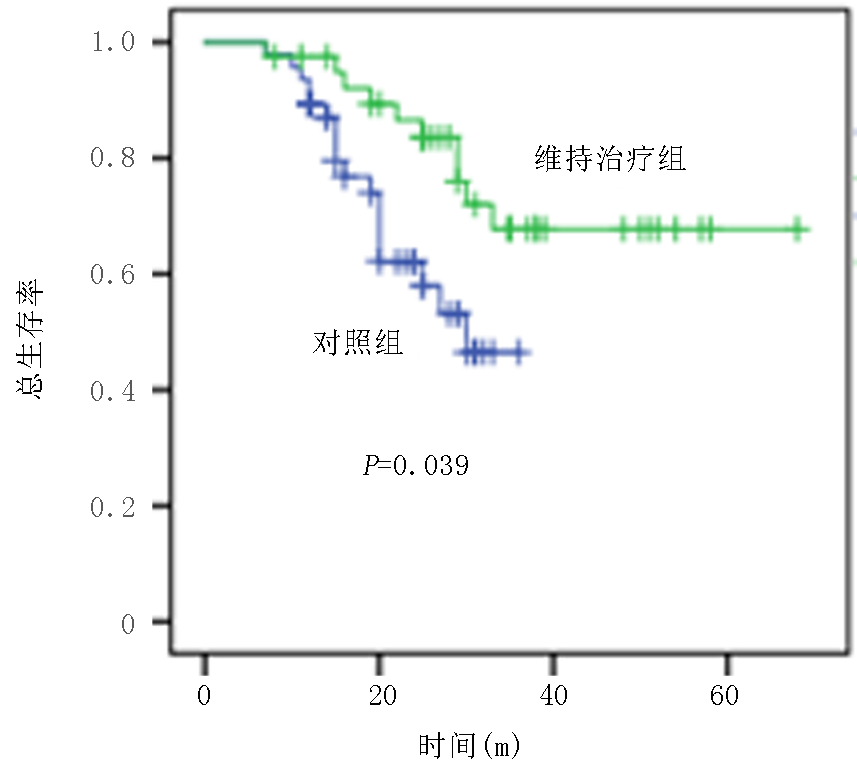
Effect of decitabine maintenance therapy on the survival of patients with low-to-medium risk of acute myeloid leukemia and suitable for intensive therapy
Zhao Yajing, Tao Qianshan, Shen Yuanyuan, Dong Yi( )
)
- Department of Hematology, the Second Affiliated Hospital of Anhui Medical University, Hefei 230601, China
-
Received:2025-01-07Online:2025-05-20Published:2025-05-23 -
Contact:Dong Yi E-mail:dongyixx@126.com
CLC Number:
Cite this article
Zhao Yajing, Tao Qianshan, Shen Yuanyuan, Dong Yi. Effect of decitabine maintenance therapy on the survival of patients with low-to-medium risk of acute myeloid leukemia and suitable for intensive therapy[J]. Clinical Focus, 2025, 40(5): 439-444.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://www.lchc.cn/EN/10.3969/j.issn.1004-583X.2025.05.010
| 项目 | 维持治疗组(n=59) | 对照组(n=48) | 统计值 | P值 |
|---|---|---|---|---|
| 性别[例(%)] | ||||
| 男 女 | 29(49.2) 30(51.8) | 24(50.0) 24(50.0) | χ2=0.087 | 0.931 |
| 预后分层[例(%)] | ||||
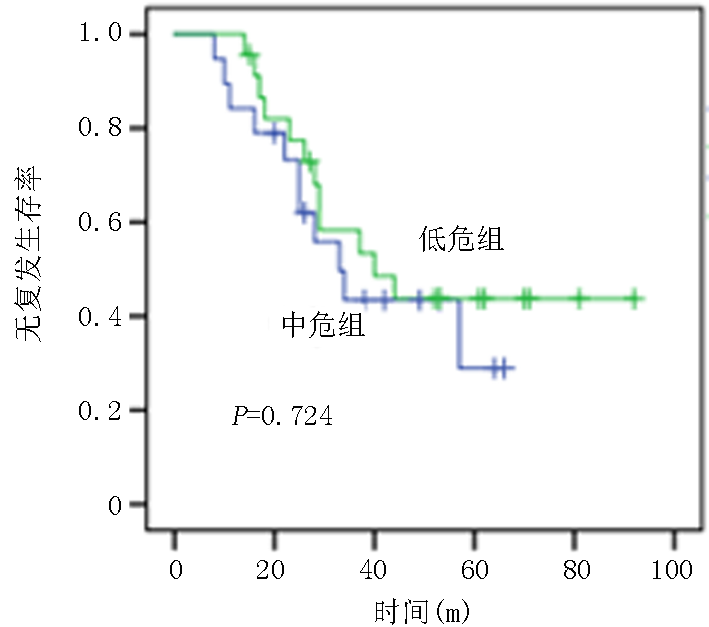
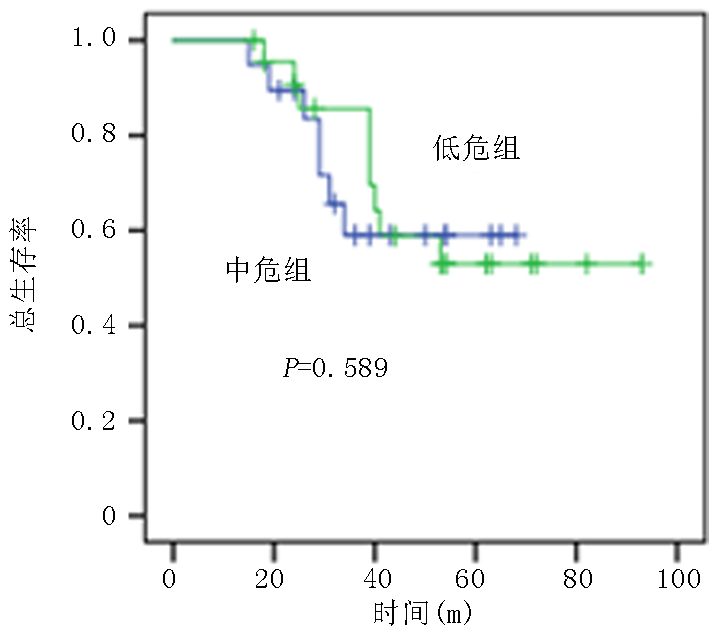
| 低危 中危 | 32(54.2) 27(45.8) | 22(45.8) 26(54.2) | χ2=0.861 | 0.389 |
| 年龄(岁) | 54(14,68) | 50(16,69) | Z=0.098 | 0.922 |
| 乳酸脱氢酶(U/L) | 323(91,1 625) | 291(26,1 426) | Z=0.915 | 0.362 |
| 原始细胞(%) | 57.00(15.00,97.00) | 56.99(21.50,97.00) | Z=0.030 | 0.976 |
| 血小板计数(×109/L) | 40(5,244) | 35(1,228) | Z=0.704 | 0.483 |
| 白细胞计数(×109/L) | 10.86(0.57,162.00) | 15.39(0.80,192.46) | Z=0.487 | 0.628 |
| 血红蛋白(g/L) | 75.0(40.0,140.0) | 80.5(23.0,141.0) | Z=0.534 | 0.594 |
Tab.1 Comparison of clinical data between the two groups
| 项目 | 维持治疗组(n=59) | 对照组(n=48) | 统计值 | P值 |
|---|---|---|---|---|
| 性别[例(%)] | ||||
| 男 女 | 29(49.2) 30(51.8) | 24(50.0) 24(50.0) | χ2=0.087 | 0.931 |
| 预后分层[例(%)] | ||||
| 低危 中危 | 32(54.2) 27(45.8) | 22(45.8) 26(54.2) | χ2=0.861 | 0.389 |
| 年龄(岁) | 54(14,68) | 50(16,69) | Z=0.098 | 0.922 |
| 乳酸脱氢酶(U/L) | 323(91,1 625) | 291(26,1 426) | Z=0.915 | 0.362 |
| 原始细胞(%) | 57.00(15.00,97.00) | 56.99(21.50,97.00) | Z=0.030 | 0.976 |
| 血小板计数(×109/L) | 40(5,244) | 35(1,228) | Z=0.704 | 0.483 |
| 白细胞计数(×109/L) | 10.86(0.57,162.00) | 15.39(0.80,192.46) | Z=0.487 | 0.628 |
| 血红蛋白(g/L) | 75.0(40.0,140.0) | 80.5(23.0,141.0) | Z=0.534 | 0.594 |
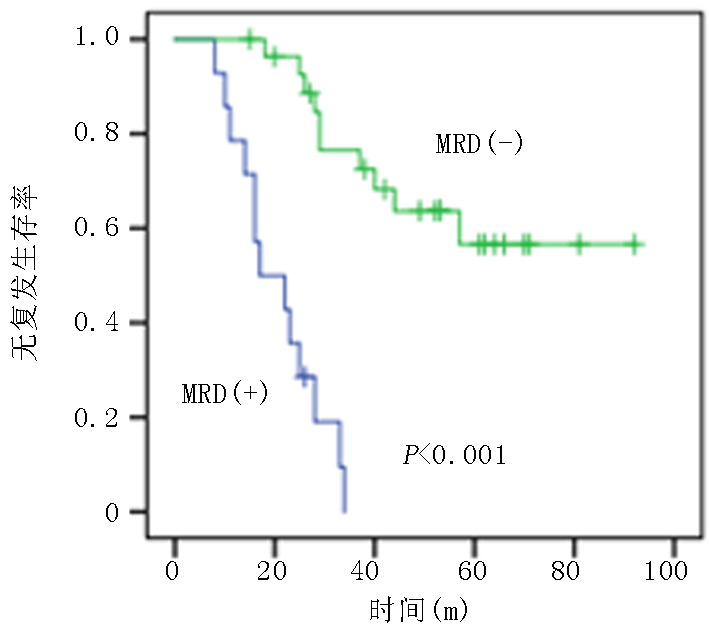
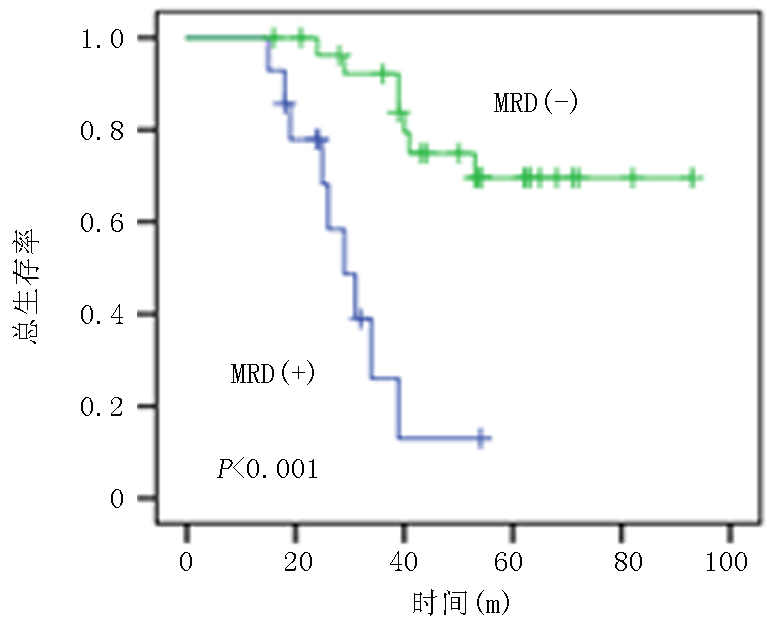
| 项目 | MRD阴性组(n=39) | MRD阳性组(n=20) | 统计值 | P值 |
|---|---|---|---|---|
| 性别[例(%)] | ||||
| 男 女 | 20(51.3) 19(48.7) | 10(50.0) 10(50.0) | χ2=0.092 | 0.926 |
| 预后分层[例(%)] | ||||
| 低危 中危 | 26(66.7) 13(33.3) | 6(30.0) 14(70.0) | χ2=2.653 | 0.008 |
| 年龄(岁) | 50(15,57) | 52(14,68) | Z=0.098 | 0.922 |
| 乳酸脱氢酶(U/L) | 312(91,1 625) | 330(125,1 228) | Z=0.576 | 0.567 |
| 原始细胞(%) | 54.00(15.00,96.00) | 61.50(21.00,97.00) | Z=0.757 | 0.452 |
| 血小板计数(×109/L) | 42(5,244) | 36.5(8,171) | Z=0.616 | 0.540 |
| 白细胞计数(×109/L) | 13.32(0.57,148.50) | 10.75(1.30,162.00) | Z=1.711 | 0.093 |
| 血红蛋白(g/L) | 77.0(40.0,133.0) | 41.5(40.0,140.0) | Z=0.194 | 0.847 |
Tab.2 Comparison of clinical data between MRD-negative group and MRD-positive group
| 项目 | MRD阴性组(n=39) | MRD阳性组(n=20) | 统计值 | P值 |
|---|---|---|---|---|
| 性别[例(%)] | ||||
| 男 女 | 20(51.3) 19(48.7) | 10(50.0) 10(50.0) | χ2=0.092 | 0.926 |
| 预后分层[例(%)] | ||||
| 低危 中危 | 26(66.7) 13(33.3) | 6(30.0) 14(70.0) | χ2=2.653 | 0.008 |
| 年龄(岁) | 50(15,57) | 52(14,68) | Z=0.098 | 0.922 |
| 乳酸脱氢酶(U/L) | 312(91,1 625) | 330(125,1 228) | Z=0.576 | 0.567 |
| 原始细胞(%) | 54.00(15.00,96.00) | 61.50(21.00,97.00) | Z=0.757 | 0.452 |
| 血小板计数(×109/L) | 42(5,244) | 36.5(8,171) | Z=0.616 | 0.540 |
| 白细胞计数(×109/L) | 13.32(0.57,148.50) | 10.75(1.30,162.00) | Z=1.711 | 0.093 |
| 血红蛋白(g/L) | 77.0(40.0,133.0) | 41.5(40.0,140.0) | Z=0.194 | 0.847 |
| 项目 | 维持治疗前 | 维持治疗后 | t值 | P值 |
|---|---|---|---|---|
| 调节T细胞(%) | 6.471±1.692 | 4.748±2.585 | 6.023 | <0.001 |
| CD8+T细胞(个/μl) | 395.2±186.64 | 570.49±457.44 | 3.178 | 0.002 |
| NK细胞(个/μl) | 126.66±85.20 | 210.86±172.63 | 4.219 | <0.001 |
Tab.3 Impact of maintenance therapy on cellular immune function
| 项目 | 维持治疗前 | 维持治疗后 | t值 | P值 |
|---|---|---|---|---|
| 调节T细胞(%) | 6.471±1.692 | 4.748±2.585 | 6.023 | <0.001 |
| CD8+T细胞(个/μl) | 395.2±186.64 | 570.49±457.44 | 3.178 | 0.002 |
| NK细胞(个/μl) | 126.66±85.20 | 210.86±172.63 | 4.219 | <0.001 |
| [1] |
Blum W, Sanford BL, Klisovic R, et al. Maintenance therapy with decitabine in younger adults with acute myeloid leukemia in first remission: A phase 2 Cancer and Leukemia Group B study (CALGB 10503)[J]. Leukemia, 2017, 31(1):34-39.
doi: 10.1038/leu.2016.252 pmid: 27624549 |
| [2] | Wei AH, Döhner H, Sayar H, et al. Long-term survival with oral azacitidine for patients with acute myeloid leukemia in first remission after chemotherapy: Updated results from the randomized, placebo-controlled, phase 3 QUAZAR AML-001 trial[J]. Am J Hematol, 2023, 98(4):E84-E87. |
| [3] | Wei AH, Dohner H, Pocock C, et al. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission[J]. N Engl J Med, 2020, 383(26):2526-2537. |
| [4] |
Roboz GJ, Ravandi F, Wei AH, et al. Oral azacitidine prolongs survival of patients with AML in remission independently of measurable residual disease status[J]. Blood, 2022, 139(14):2145-2155.
doi: 10.1182/blood.2021013404 pmid: 34995344 |
| [5] |
Dong Y, Wang J, Tao QS, et al. Effectiveness and mechanism of decitabine maintenance therapy in patients with medium and low-risk acute myeloid leukemia[J]. J Exp Hematol, 2022, 30(5):1369-1375.
doi: 10.19746/j.cnki.issn.1009-2137.2022.05.011 pmid: 36208237 |
| [6] | Dohner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN[J]. Blood, 2022, 140(12):1345-1377. |
| [7] | Cooperrider JH, Shukla N, Nawas MT, et al. The cup runneth over: Treatment strategies for newly diagnosed acute myeloid leukemia[J]. JCO Oncol Pract, 2023, 19(2):74-85. |
| [8] |
Short NJ, Zhou S, Fu C, et al. Association of measurable residual disease with survival outcomes in patients with acute myeloid leukemia: A systematic review and meta-analysis[J]. JAMA Oncol, 2020, 6(12):1890-1899.
doi: 10.1001/jamaoncol.2020.4600 pmid: 33030517 |
| [9] |
Kantarjian H, Kadia T, DiNardo C, et al. Acute myeloid leukemia: Current progress and future directions[J]. Blood Cancer J, 2021, 11(2):41.
doi: 10.1038/s41408-021-00425-3 pmid: 33619261 |
| [10] |
Toksvang LN, Lee SHR, Yang JJ, et al. Maintenance therapy for acute lymphoblastic leukemia: Basic science and clinical translations[J]. Leukemia, 2022, 36(7):1749-1758.
doi: 10.1038/s41375-022-01591-4 pmid: 35654820 |
| [11] |
Sanz MA, Fenaux P, Tallman MS, et al. Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European LeukemiaNet[J]. Blood, 2019, 133(15):1630-1643.
doi: 10.1182/blood-2019-01-894980 pmid: 30803991 |
| [12] | Kadia TM, Reville PK, Wang X, et al. Phase II study of venetoclax added to cladribine plus low-dose cytarabine alternating with 5-azacitidine in older patients with newly diagnosed acute myeloid leukemia[J]. J Clin Oncol, 2022, 40(33):3848-3857. |
| [13] |
Wei AH, Panayiotidis P, Montesinos P, et al. Long-term follow-up of VIALE-C in patients with untreated AML ineligible for intensive chemotherapy[J]. Blood, 2022, 140(25):2754-2756.
doi: 10.1182/blood.2022016963 pmid: 36112968 |
| [14] |
Senapati J, Kadia TM, Ravandi F. Maintenance therapy in acute myeloid leukemia: Advances and controversies[J]. Haematologica, 2023, 108(9):2289-2304.
doi: 10.3324/haematol.2022.281810 pmid: 37139599 |
| [15] |
Huls G, Chitu DA, Havelange V, et al. Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients[J]. Blood, 2019, 133(13):1457-1464.
doi: 10.1182/blood-2018-10-879866 pmid: 30630862 |
| [16] | Wei Y, Xiong X, Li X, et al. Low-dose decitabine plus venetoclax is safe and effective as post-transplant maintenance therapy for high-risk acute myeloid leukemia and myelodysplastic syndrome[J]. Cancer Sci, 2021, 112(9):3636-3644. |
| [17] | Bazinet A, Kantarjian H, Bataller A, et al. Reduced dose azacitidine plus venetoclax as maintenance therapy in acute myeloid leukaemia following intensive or low-intensity induction: A single-centre, single-arm, phase 2 trial[J]. Lancet Haematol, 2024, 11(4):e287-e298. |
| [18] |
Platzbecker U, Middeke JM, Sockel K, et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): An open-label, multicentre, phase 2 trial[J]. Lancet Oncol, 2018, 19(12):1668-1679.
doi: S1470-2045(18)30580-1 pmid: 30442503 |
| [19] |
Venditti A, Piciocchi A, Candoni A, et al. GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia[J]. Blood, 2019, 134(12):935-945.
doi: 10.1182/blood.2018886960 pmid: 31395600 |
| [20] | Zhao Y, Guo H, Chang Y. MRD-directed and risk-adapted individualized stratified treatment of AML[J]. Chin J Cancer Res, 2023, 35(5):451-469. |
| [21] |
Wang D, Sun Z, Zhu X, et al. GARP-mediated active TGF-β1 induces bone marrow NK cell dysfunction in AML patients with early relapse post-allo-HSCT[J]. Blood, 2022, 140(26):2788-2804.
doi: 10.1182/blood.2022015474 pmid: 35981475 |
| [22] |
Jia XY, Yang WZ, Zhou XH, et al. Influence of demethylation on regulatory T and Th17 cells in myelodysplastic syndrome[J]. Oncol Lett, 2020, 19(1):442-448.
doi: 10.3892/ol.2019.11114 pmid: 31897157 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||